|
|
21 CFR 820.100(a)(3) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action. The procedures shall include requirements for:
(3) Identifying the action(s) needed to correct and prevent recurrence of nonconforming product and other quality problems.
|
CAPA Identification Phase The regulatory requirement is to identify and capture all Corrective and Preventive actions necessary to address and prevent the causal factors identified in the Investigation Phase. The CAPA plan must clearly define each action and explain how it corrects or prevents recurrence of the problem or issue.
It is important to remember that corrective actions should be sustainable, practical solutions to a non-conformity and their implementation is time bound.
The ExtraView Difference
The ExtraView Enterprise solution enables corrective and preventive action identification within the surrounding workflow required by your company's practices. Users are able to establish parent-child and peer relationships between CAPAs and manage them accordingly, either directly or through our robust reporting features. Within ExtraView Enterprise, you can capture the expected result of each corrective and preventive action and how it addresses the root cause(s) effectively and completely.
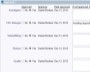
At this stage, you have richer information and may want to perform a second risk assessment or an update to the original risk assessment. This will enable prioritization of individual CAPAs as part of the holistic system. ExtraView automatically provides a clear audit trail of updates and modifications to any record. This information is easily seen within the application and through reporting as desired.
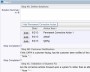
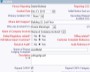
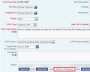
Example Screens from Customer Implementations
Learn About Other CAPA Phases Please select the Verify and Validate link to move to the next CAPA phase, or select any of the puzzle pieces below to learn more about how ExtraView meets your business requirements in that specific CAPA area. For a synopsis of ExtraView’s role in the entire CAPA process, see the ExtraView CAPA Functionality Matrix.
|
|




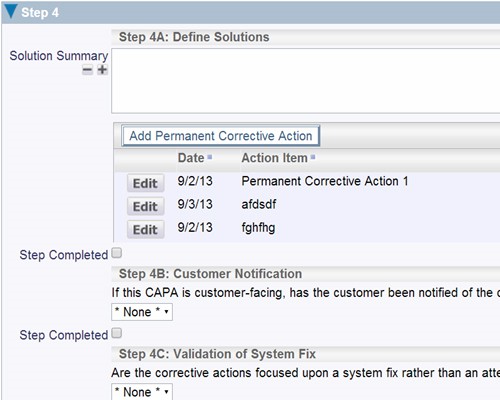 Create detailed descriptions and relationships between actions
Create detailed descriptions and relationships between actions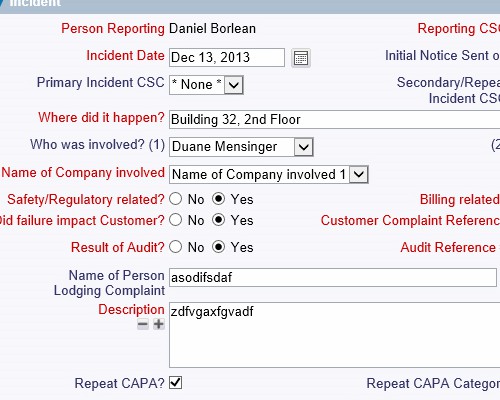 Configure screens that reflect the way your organization works
Configure screens that reflect the way your organization works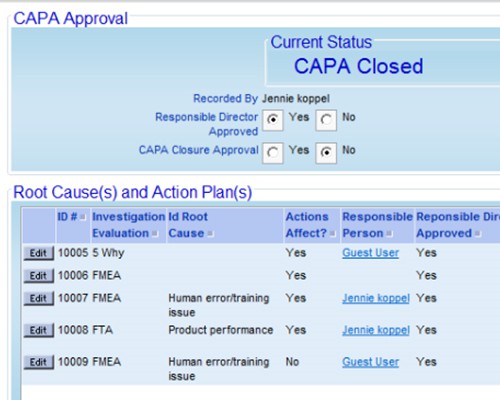 Capture evidence of accountability, ownership and oversight
Capture evidence of accountability, ownership and oversight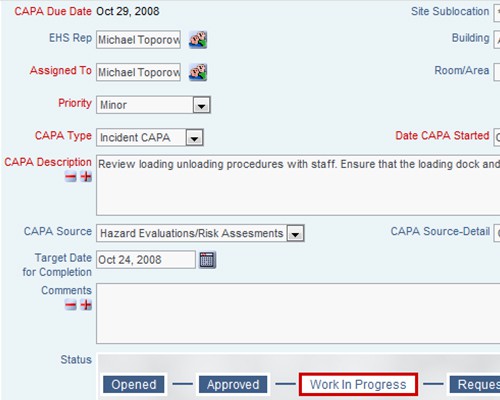 Define the required actions and assign responsibility with deadlines
Define the required actions and assign responsibility with deadlines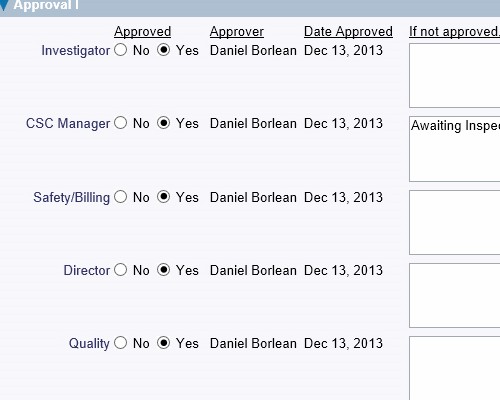 Leverage auditable electronic signatures for internal review and signoff at any step in the process
Leverage auditable electronic signatures for internal review and signoff at any step in the process