|
CAPA Within a Quality Management Framework
CAPA is one of the most critical components of a comprehensive Quality Management system. It is at the core of quality, hence its close scrutiny from regulatory bodies such as the FDA. This diagram represents how CAPA fits within the framework:
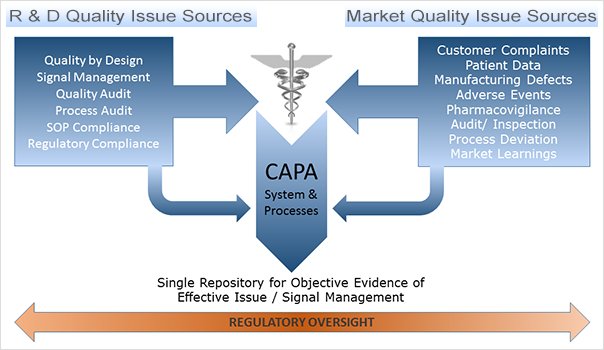
CAPA Within a Quality Management Framework
The ExtraView Difference
The ExtraView Enterprise platform is an affordable, enterprise software solution that manages your CAPA process, automates workflow and provides on-going monitoring, while giving objective evidence of compliance and proactive management.
ExtraView Enterprise is fully customizable to meet your business needs and easily integrates with any existing quality, defect and complaint management systems. ExtraView excels in providing you with a solution that follows your processes and your workflows, with simple tools to implement your requirements within a fully auditable, verifiable and validated environment. Moreover, ExtraView includes a complete set of report types, all easily set up without the need for IT expertise.
Please click on a puzzle piece or a link below to learn more about how ExtraView Enterprise meets your business requirements in any particular CAPA area, or see the ExtraView CAPA Functionality Matrix for a quick synopsis.
|
