|
|
21 CFR 820.100(a)(6) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action. The procedures shall include requirements for:
(6) Ensuring that information related to quality problems or nonconforming product is disseminated to those directly responsible for assuring the quality of such product or the prevention of such problems.
|
CAPA Dissemination Phase Dissemination addresses:
Dissemination is the requirement to communicate information on the CAPA, including risks, remediation plans, and training to all responsible, affected, and interested parties throughout the lifespan of the CAPA.
The EU Guideline on good pharmacovigilance practices (GVP) – Module I EMA/541760/2011, characterizes the training requirement in this way, "Adequate training should also be considered by the organisation for those staff members to whom no specific pharmacovigilance tasks and responsibilities have been assigned but whose activities may have an impact on the pharmacovigilance system or the conduct of pharmacovigilance. Such activities include but are not limited to those related to clinical trials, technical product complaints, medical information, terminologies, sales and marketing, regulatory affairs, legal affairs and audits." A similar model applies to all quality processes.
The ExtraView Difference
ExtraView Enterprise actively manages and tracks CAPA Dissemination workflow from the Analysis Phase through to CAPA close.
Training records - including metrics on the effectiveness of training, process documents, and other relevant information can be stored in ExtraView directly, or in your source repository with a link back to ExtraView Enterprise, along with an acknowledgement that the workflow steps were completed. ExtraView keeps all stakeholders in the loop with continual email notifications, scheduled reports, interest lists, escalation rules and more.
Please select this link to move to the next CAPA phase, Management Review. Select any of the puzzle pieces below to learn more about how ExtraView meets your business requirements in that specific CAPA area. For a synopsis of ExtraView’s role in the entire CAPA process, see the ExtraView CAPA Functionality Matrix.
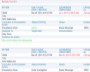
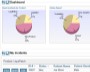
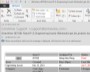
Example Screens from Customer Implementations
Learn About Other CAPA Phases
|
|





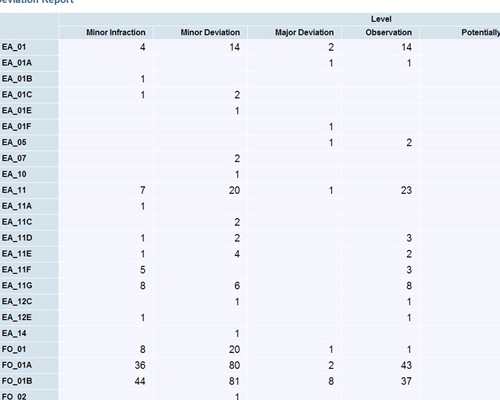 Easily create, publish and share reports
Easily create, publish and share reports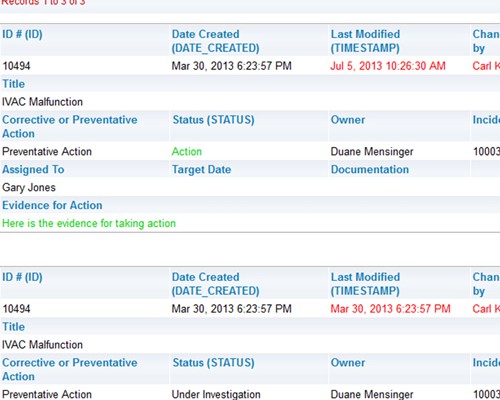 Shared audit trails so all stakeholders can view CAPA progress
Shared audit trails so all stakeholders can view CAPA progress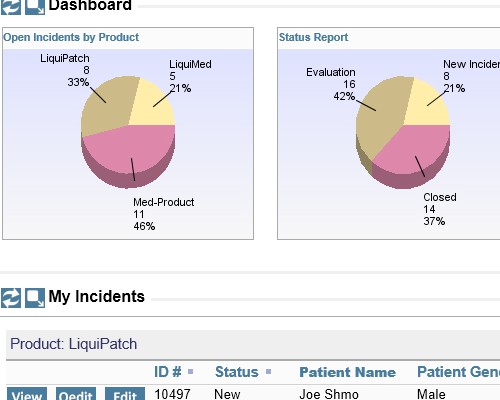 Share information with your peer group
Share information with your peer group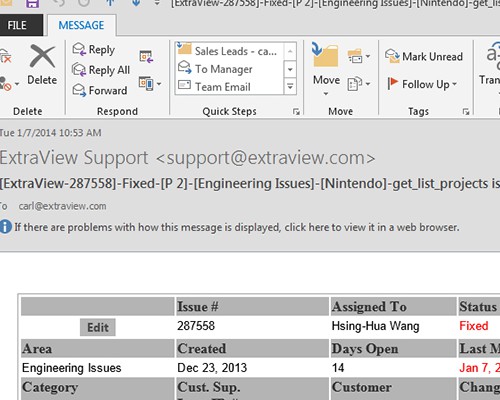 All interested parties receive immediate notification in email
All interested parties receive immediate notification in email Leverage relationships between issues and CAPAs to look for unidentified signals or trends
Leverage relationships between issues and CAPAs to look for unidentified signals or trends