|
|
21 CFR 820.100(b) (b) All activities required under this section, and their results, shall be documented.
|
CAPA Documentation Phase
The CAPA Documentation Phase is the requirement around creating and preserving objective evidence. The existence of a well formed, well documented CAPA system is critical to a regulator's assessment of a manufacturer's seriousness in addressing quality issues / signal and risk management. Where evidence has lacked, regulators actions have been more severe.
The EU Guideline on Pharmacovigilance, Section 1.B.11 Documentation of the quality system – which includes CAPA, states, "All elements, requirements and provisions adopted for the quality system shall be documented in a systematic and orderly manner in the form of written policies and procedures, such as quality plans, quality manuals and quality records [IR Art 8(4)]."
The ExtraView Difference
By managing your CAPA process in ExtraView Enterprise, you have a single repository to provide transparency to regulators and auditors that you are proactively managing quality within your organization. The ExtraView CAPA system provides the evidence that the required steps were followed and that the appropriate accountabilities were assigned, managed, and monitored.
ExtraView Enterprise can maintain all documentation within its own database, or can provide access to documents stored within a separate documentation repository, whether on your own servers, or stored in the cloud.
Select any of the puzzle pieces below to learn more about how ExtraView meets your business requirements in that specific CAPA area. For a synopsis of ExtraView’s role in the entire CAPA process, see the ExtraView CAPA Functionality Matrix.
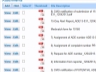
Example Screens from Customer Implementations
Learn About Other CAPA Phases
|
|





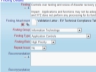
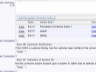
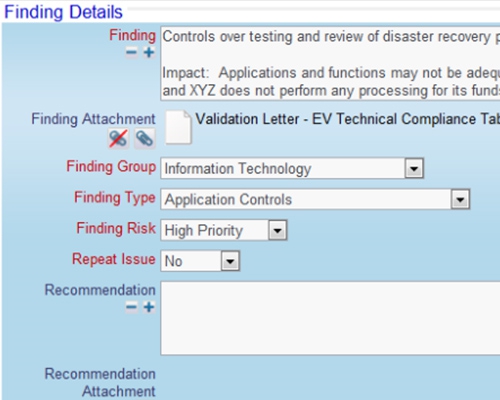 Documents are controlled with workflow
Documents are controlled with workflow SOPs may be stored in ExtraView or any external system
SOPs may be stored in ExtraView or any external system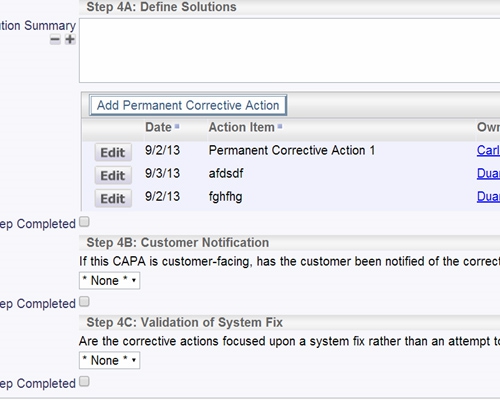 Store documents within ExtraView or in an external document repository
Store documents within ExtraView or in an external document repository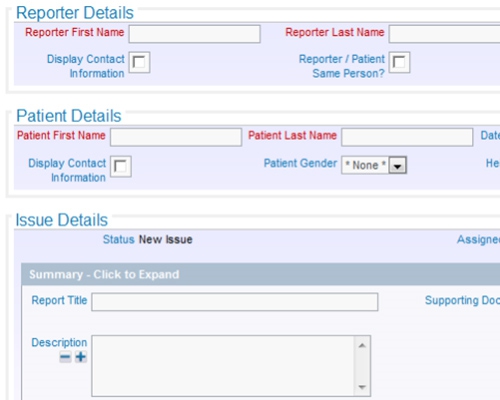 All data entered can be a reference and a documented audit trail
All data entered can be a reference and a documented audit trail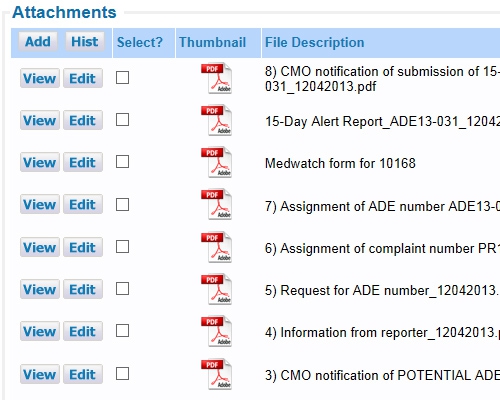 Include or point to any number of documents
Include or point to any number of documents