|
|
21 CFR 820.100(a)(5) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action. The procedures shall include requirements for:
(5) Implementing and recording changes in methods and procedures needed to correct and prevent identified quality problems.
|
CAPA Implementation Phase The CAPA Implementation phase involves executing the plan:
Documentation, as part of implementation, may include new or updated:
The ExtraView Difference Organizations may choose to maintain each document in its respective source system (where applicable) and a record of the change / reference to the source document in Extraview. You may also choose to store the documents in ExtraView. In each case, ExtraView Enterprise remains the authoritative record of change associated with the CAPA.
After implementation, there may also be a second Verification and Validation phase.
Please select the Disseminate link to move to the next CAPA phase, or select any of the puzzle pieces below to learn more about how ExtraView meets your business requirements in that specific CAPA area. For a synopsis of Extraview’s role in the entire CAPA process, see the ExtraView CAPA Functionality Matrix.
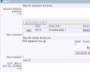
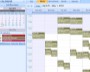
Example Screens from Customer Implementations
Learn About Other CAPA Phases
|
|




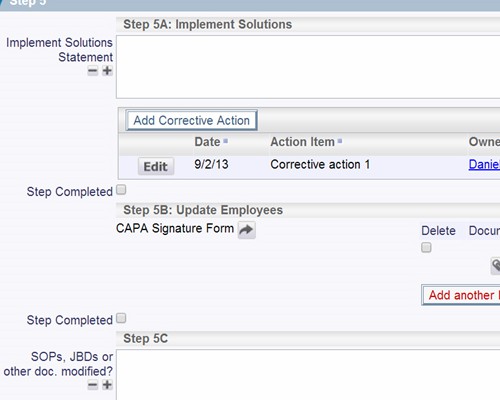 Follow the workflow you define, with full audit trails
Follow the workflow you define, with full audit trails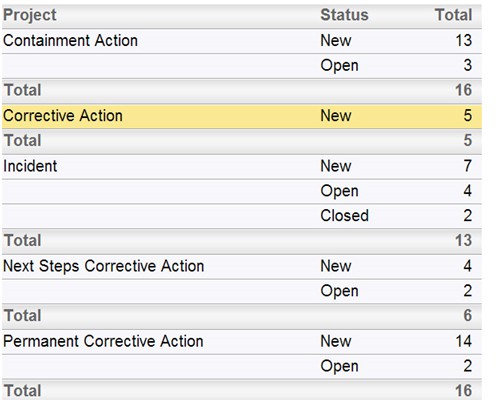 Instant summarizations, with drilldown into details
Instant summarizations, with drilldown into details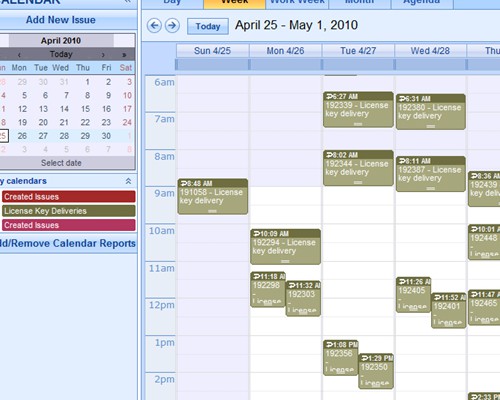 Report using interactive calendars to schedule implementations and monitor performance against plan
Report using interactive calendars to schedule implementations and monitor performance against plan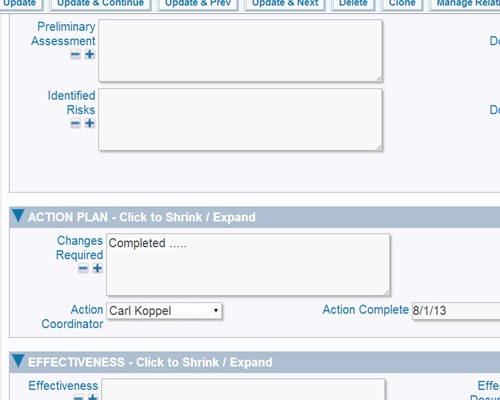 Validate plans for effectiveness before implementation
Validate plans for effectiveness before implementation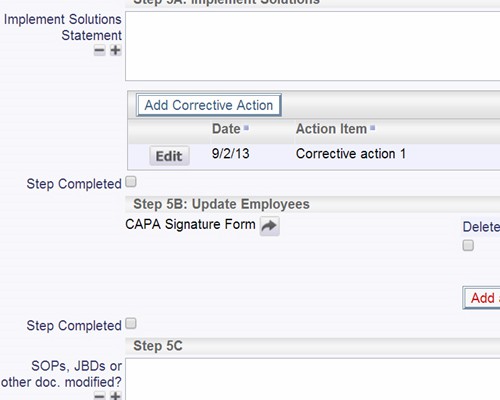 SOP changes and updates can be part of the process
SOP changes and updates can be part of the process